News
-
9 2021
Venus Sosa Iglesias has joined the lab! Scott Atwood
 Venus, a brilliant postdoctoral scholar, has decided to pursue her training in our lab. Venus received her B.S. from the University of Lleida in Spain and her Ph.D. in Radiation Oncology
from University of Maastricht in the Netherlands where she studied Notch inhibitor therapy as a non-small cell lung cancer treatment. Venus is excited to work
on transcription factor regulation in skin development and basal cell carcinoma. Welcome to the lab!
Venus, a brilliant postdoctoral scholar, has decided to pursue her training in our lab. Venus received her B.S. from the University of Lleida in Spain and her Ph.D. in Radiation Oncology
from University of Maastricht in the Netherlands where she studied Notch inhibitor therapy as a non-small cell lung cancer treatment. Venus is excited to work
on transcription factor regulation in skin development and basal cell carcinoma. Welcome to the lab!
-
7 2021
Scott has been granted tenure and a promotion to Associate Professor! Scott Atwood
This remarkable achievement could not have been possible without the exceptional people within the Atwood lab. Their tireless efforts to push the boundaries of science have collectively made some groundbreaking achievements in stem cell and cancer biology that will propel this lab and others forward into the unknown. Mentors were also vital during this maturation process and the outstanding investigators within the Developmental and Cell Biology Department, the skin community, and systems biology at UC Irvine were essential in guiding my way forward towards success. Family support is the cornerstone to my success and I can't thank them enough for their generosity in time and temperment. It takes a village to raise a scientist and I'm proud to be a part of an amazing community that cares about the success of their people and I can't wait to pay it forward!
-
7 2021
Adam, Emmanuel, and Kirsten have been awarded the CMCF fellowship! Scott Atwood
The NSF-Simons Center for Multiscale Cell Fate Research (CMCF) is connecting scientists across mathematical, physical, and biological sciences to usher in a new era of biology. CMCF is one of four NSF-Simons Research Centers for Mathematics of Complex Biological Systems situated across the country, jointly funded by the National Science Foundation and the Simons Foundation. CMCF takes on the formidable multiscale challenges associated with investigating complex cell fate systems using an integrated mathematical and experimental approach.
-
6 2021
Frontiers in Oncology published our work showing PI3K inhibition promotes p21 stability to suppress basal cell carcinoma Scott Atwood
 Basal cell carcinoma (BCC) is a locally invasive epithelial cancer that is primarily driven by the Hedgehog (HH) pathway. Advanced BCCs are a critical subset of BCCs that frequently acquires
resistance to Smoothened (SMO) inhibitors and identifying pathways that bypass SMO could provide alternative treatments for patients with advanced or metastatic BCC. Here, we use a combination of RNA-sequencing analysis of advanced human BCC tumor-normal pairs and immunostaining
of human and mouse BCC samples to identify a PI3K pathway expression signature in BCC. Pharmacological inhibition of PI3K activity in BCC cells significantly reduces cell proliferation and HH signaling. However, treatment of Ptch1fl/fl; Gli1-CreERT2 mouse BCCs with the PI3K
inhibitor BKM120 results in a reduction of tumor cell growth with no significant effect on HH signaling. Downstream PI3K components aPKC and Akt1 showed a reduction in active protein, whereas their substrate, cyclin-dependent kinase inhibitor p21, showed a concomitant increase
in protein stability. Our results suggest that PI3K promotes BCC tumor growth by kinase-induced p21 degradation without altering HH signaling.
Read More PDF
Basal cell carcinoma (BCC) is a locally invasive epithelial cancer that is primarily driven by the Hedgehog (HH) pathway. Advanced BCCs are a critical subset of BCCs that frequently acquires
resistance to Smoothened (SMO) inhibitors and identifying pathways that bypass SMO could provide alternative treatments for patients with advanced or metastatic BCC. Here, we use a combination of RNA-sequencing analysis of advanced human BCC tumor-normal pairs and immunostaining
of human and mouse BCC samples to identify a PI3K pathway expression signature in BCC. Pharmacological inhibition of PI3K activity in BCC cells significantly reduces cell proliferation and HH signaling. However, treatment of Ptch1fl/fl; Gli1-CreERT2 mouse BCCs with the PI3K
inhibitor BKM120 results in a reduction of tumor cell growth with no significant effect on HH signaling. Downstream PI3K components aPKC and Akt1 showed a reduction in active protein, whereas their substrate, cyclin-dependent kinase inhibitor p21, showed a concomitant increase
in protein stability. Our results suggest that PI3K promotes BCC tumor growth by kinase-induced p21 degradation without altering HH signaling.
Read More PDF
-
5 2021
Congratulations to Dr. Rachel Chow on defending her thesis! Scott Atwood
 Rachel showed remarkable resilience in successfully defending her thesis during these uncertain times. She displayed a strong grasp of drug screening in basal cell carcinoma,
showing the myriad ways she investigated potential therapeutic options to treat this cancer.
Rachel showed remarkable resilience in successfully defending her thesis during these uncertain times. She displayed a strong grasp of drug screening in basal cell carcinoma,
showing the myriad ways she investigated potential therapeutic options to treat this cancer.
Rachel was in the first cohort of graduate students to join the lab and her drug screens touched many projects to provide important preclinical discoveries. Rachel dissected how suppressing putative non-canonical Hedgehog pathway components influenced basal cell carcinoma growth. She demonstrated how the PI3K-PDK1-MTOR, SRC, and aPKC pathways largely suppressed tumor growth by hitting different points of the Hedgehog pathway. Born out of a desire to target aPKC, a GLI1 kinase necessary for tumor growth, Rachel meticulously screened through drugs that target a host of putative aPKC activators and was able to show how these drugs acted in both cancer cells and in vivo tumors. Interestingly, some of the targets acted quite differently when pharmacologially suppressed in cell culture versus when the microenvironment was present, important lessons to learn when discovering new cancer therapies.
Rachel's future holds the promise of more discoveries to improve patient outcomes and we wish her the best of luck on her new adventures! -
3 2021
Congratulations to Dr. Tuyen Nguyen on defending her thesis! Scott Atwood
 Despite the COVID pandemic and research restrictions for over a year, Tuyen successfully defended her thesis. With a remarkable presentation, she calmed the turbulent waters
and showed her mastery of aPKC and PKA in basal cell carcinoma progression and drug resistance. Nearly stunned to silence, the committee unanimously approved her Ph.D. and was happy to call her Dr. Nguyen.
Despite the COVID pandemic and research restrictions for over a year, Tuyen successfully defended her thesis. With a remarkable presentation, she calmed the turbulent waters
and showed her mastery of aPKC and PKA in basal cell carcinoma progression and drug resistance. Nearly stunned to silence, the committee unanimously approved her Ph.D. and was happy to call her Dr. Nguyen.
Tuyen was one of the first graduate students to join the lab and she left her indelible mark on it forever. A joy to work with, Tuyen pushed the boundaries of knowledge and revealed the inner workings of how basal cell carcinomas can persist in the face of therapeutic treatments. She characerized a unique isoform of aPKC that promotes Hedgehog signaling in the presence or absence of primary cilia. She also showed that it's expression promotes drug resistance to current Smoothened inhibitors that are used in the clinic. In addition, Tuyen upended the dogma in the Hedgehog signaling field on how GLI transcription factors are processed. She discovered that GLI1 is phosphorylated by PKA, which facilitates GLI1 cleavage in a BTRC-dependent process. One of these discoveries would be enough to cement her name in the annals of scientific research history, not to mention two.
Although she will be missed, Tuyen's future is bright and we look forward to the mark she will leave as she ventures into new opportunities. Good luck on your new adventures and know that we will always be supporting you! -
3 2021
Experimental Dermatology published our editorial on skin organoid cultures Scott Atwood
-
3 2021
Experimental Dermatology published our work on MTOR inhibition as a therapeutic option in basal cell carcinoma Scott Atwood
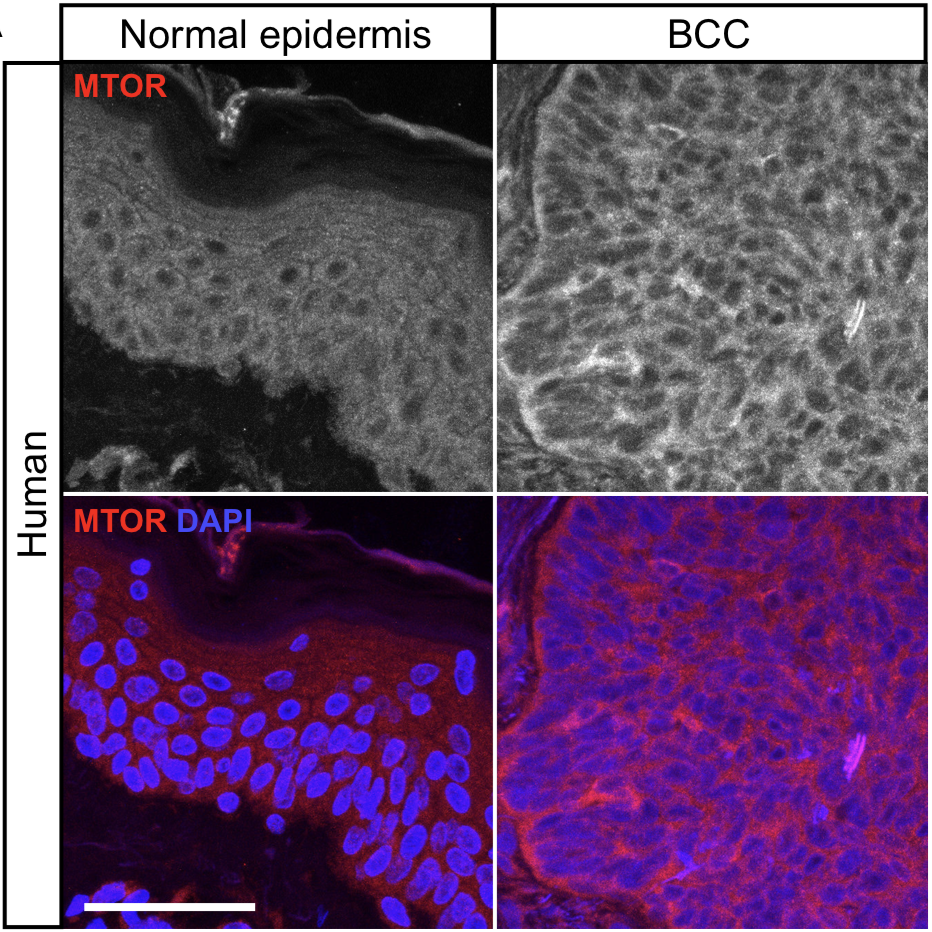 Advanced basal cell carcinomas (BCCs) are driven by the Hedgehog (HH) pathway and often possess inherent resistance to SMO inhibitors. Identifying and targeting
pathways that bypass SMO could provide alternative treatments for patients with advanced or metastatic BCC. Here, we use a combination of RNA-sequencing analysis of advanced human BCC tumor-normal pairs and immunostaining of human and mouse BCC samples
to identify an MTOR expression signature in BCC. Pharmacological inhibition of MTOR activity in BCC cells significantly reduces cell proliferation without affecting HH signaling. Similarly, treatment of the Ptch1fl/fl; Gli1-CreERT2 mouse BCC tumor model
with everolimus reduces tumor growth. aPKC, a downstream target of MTOR, shows reduced activity, suggesting that MTOR promotes tumor growth by activating aPKC and demonstrating that suppressing MTOR could be a promising target for BCC patients.
Read More PDF bioRxiv
Advanced basal cell carcinomas (BCCs) are driven by the Hedgehog (HH) pathway and often possess inherent resistance to SMO inhibitors. Identifying and targeting
pathways that bypass SMO could provide alternative treatments for patients with advanced or metastatic BCC. Here, we use a combination of RNA-sequencing analysis of advanced human BCC tumor-normal pairs and immunostaining of human and mouse BCC samples
to identify an MTOR expression signature in BCC. Pharmacological inhibition of MTOR activity in BCC cells significantly reduces cell proliferation without affecting HH signaling. Similarly, treatment of the Ptch1fl/fl; Gli1-CreERT2 mouse BCC tumor model
with everolimus reduces tumor growth. aPKC, a downstream target of MTOR, shows reduced activity, suggesting that MTOR promotes tumor growth by activating aPKC and demonstrating that suppressing MTOR could be a promising target for BCC patients.
Read More PDF bioRxiv
-
1 2021
Chan Hong has joined the lab! Scott Atwood
 Chan, a brilliant master's student from the Biotechnology program, has decided to pursue her master's work in the lab. Chan received her B.S. in Biochemistry from Lehigh
University where she worked on membrane protein structure and function. Chan is excited to expand her interests by defining how primary cilia function during epidermal hyperplasia. Welcome to the lab!
Chan, a brilliant master's student from the Biotechnology program, has decided to pursue her master's work in the lab. Chan received her B.S. in Biochemistry from Lehigh
University where she worked on membrane protein structure and function. Chan is excited to expand her interests by defining how primary cilia function during epidermal hyperplasia. Welcome to the lab!
- Previous Older posts
