How do cells choose their fate?
Our Science
The Atwood lab is interested in how stem cell heterogeneity drives epidermal homeostasis and disease. We focus on protein kinase regulation of signaling pathways and transcription factors, using models of skin development and basal cell carcinoma, to determine how kinases influence cell fate by controlling the genetic landscape of the cell.
Recent Work
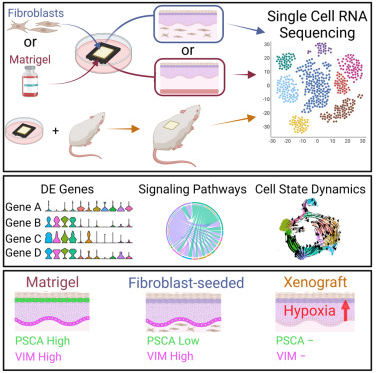 Single cell transcriptomics of human skin equivalent organoids
Single cell transcriptomics of human skin equivalent organoids
Several methods for generating human skin equivalent (HSE) organoid cultures are in use to study skin biology, however, few studies thoroughly characterize these systems. To fill this gap, we use single cell transcriptomics to compare in vitro HSEs, xenograft HSEs, and in vivo epidermis. By combining differential gene expression, pseudotime analyses, and spatial localization, we reconstruct HSE keratinocyte differentiation trajectories that recapitulate known in vivo epidermal differentiation pathways and show that HSEs contain major in vivo cellular states. However, HSEs also develop unique keratinocyte states, an expanded basal stem cell program, and disrupted terminal differentiation. Cell-cell communication modeling shows aberrant EMT-associated signaling pathways that alter upon EGF supplementation. Lastly, xenograft HSEs at early timepoints post-transplantation significantly rescue many in vitro deficits, while undergoing a hypoxic response that drives an alternative differentiation lineage. This study highlights the strengths and limitations of organoid cultures and identifies areas for potential innovation.
Cell Reports. 2023. 42(5):112511.
Link PDF
bioRxiv